EVerGel
EVERGel is being developed in two gastrointestinal healing indications that represent significant unmet medical needs and a very poor quality of life for patients.
Complex perianal fistula

Crohn’s disease is a lifelong inflammatory bowel disease that is most common in Western Europe and North America, where it has a prevalence of 100 to 300 per 100,000 people. Approximately one in four people with Crohn’s disease will develop a fistula during their lifetime.
Perianal fistulas are considered “complex”, when they:
- Involve a large portion of the sphincter muscle that opens and closes the anus.
- Branch or have more than one opening into muscle or skin.
- Become infected or form a pus-filled sac inside called an abscess.
- Involve the rectum or vagina.
- Occur in a person with inflammatory bowel disease, fecal incontinence, chronic diarrhea, or anorectal cancer.
This complication requires both medical (antibiotic therapy, biotherapy) and surgical (drainage and obturation of fistulous tracts) management. However, despite multidisciplinary management, the rate of therapeutic failure remains high, with 62% of patients failing to achieve complete remission.
Anastomotic healing

Tumor resection surgery is required in over 75% of cases of colorectal and esophageal cancer, representing more than 2M patients undergo surgery worldwide each year. Around 10% (180k patients) will suffer serious complications caused by a failure of the surgical connection between the two tubular structures (anastomotic leak) and may require extensive care. About 3% of them may not survive this complication.
EVerGel proof-of-concept
EVerGel is an exosome based product delivered via a thermoresponsive hydrogel to promoting controlled release and favoring retention at the site of interest.
EVerGel is composed of exosomes produced from human adipose stem cells using our proprietary cGMP turbulent process, embedded in a hydrogel. The gel is directly injected onto the injury site in the liquid state <20°C and gelation occurs in situ at body temperature. Therefore, the hydrogel offer the advantage of filling irregular defects, reaching low-accessible areas, promote an occlusive effect and retain exosomes in the injury site.
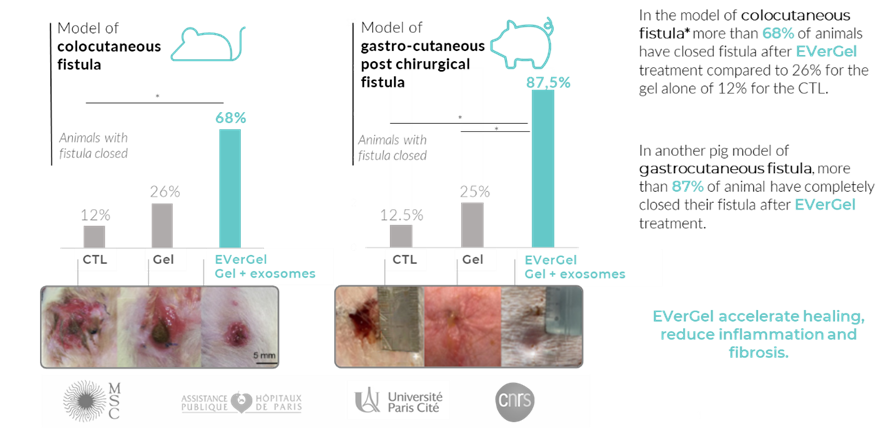
EVerGel has demonstrated promising preclinical in vitro and in vivo fistula proof-of-concept data. A total of 8 different models* (3 pig models and 5 mouse models) were tested to demonstrate the efficacy and versatility of exosomes.
First-in-human studies in both anastomosis healing and Crohn’s disease-associated fistula indications are planned for early 2026.
* A.K.A. Silva et al., Thermoresponsive Gel Embedding Adipose Stem Cell- Derived Extracellular Vesicles Promotes Esophageal Fistula Healing in a Thermo-Actuated Delivery Strategy, ACS Nano, 2018
A. Berger et al., Local administration of stem cell-derived extracellular vesicles in a thermoresponsive hydrogel promotes a pro-healing effect in a rat model of colo-cutaneous post-surgical fistula, Nanoscale, 2021
E. Coffin et al., Extracellular vesicles from adipose stromal cells combined with a thermoresponsive hydrogel prevent esophageal stricture after extensive endoscopic submucosal dissection in a porcine model, Nanoscale, 2021