Exosomes : nano-messengers in intercellular communication
Nomenclature and history of exosomes
According to the International Society for Extracellular Vesicles (or EVs) are ubiquitous nanoparticles naturally released from cells, delimited by a lipid bilayer and that cannot replicate (Théry et al. 2018). While they were originally considered as cellular debris, they became more and more interesting over the years. In 1967, Wolf investigated the material responsible for coagulation: a small particle rich in lipid content, originating from platelets described as “platelet-dust” (Wolf 1967).
It is only in 1971, that Aaronson et al. were the first to use the term “extracellular vesicles” (Aaronson et al. 1971). In the 90’s, Raposo et al. demonstrated that extracellular vesicles are actors in the immune system by inducing T cells response (Raposo et al. 1996). Later, several studies showed that proteins and ribonucleic acid (RNA) were transferred from cell to cell through extracellular vesicles (Ratajczak et al. 2006; Skog et al. 2008).
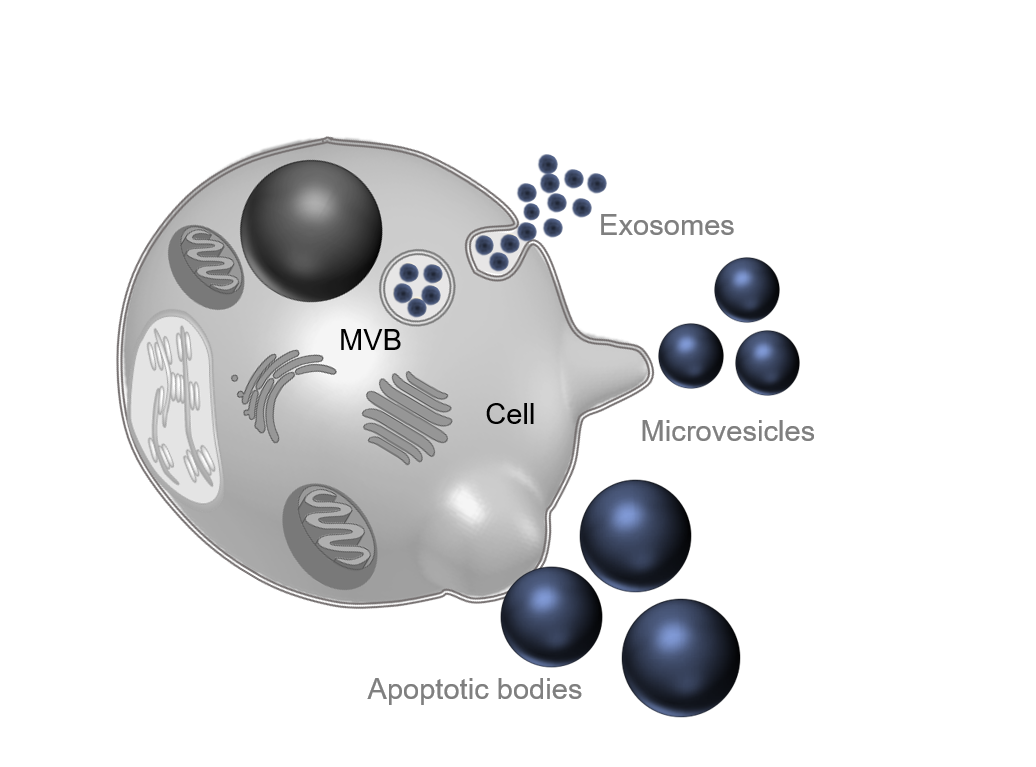
Empirically, different subtypes of extracellular vesicles were distinguished according to their size, composition, and biogenesis: exosomes (less than 150 nm), microvesicles or ectosomes (between 50 nm and 500 nm), and apoptotic bodies (until 2,000 nm) (EL Andaloussi et al. 2013). However, this nomenclature has been reviewed after the MISEV 2018 guidelines. Now, it is common to use operational terms that can be experimentally verified to define extracellular vesicles (Théry et al. 2018). On this page, the term “extracellular vesicles” will be used to include all the EV subtypes.
Biological composition and biogenesis
Extracellular vesicles are enriched in proteins, nucleic acids and lipids. Proteins commonly found on the surface and used as extracellular vesicle markers are tetraspanins (CD9, CD63, CD81, CD82) and major histocompatibility complex. Enzymes and cytosolic proteins (HSP70, HSP90, Alix, TSG101, Rab) are present inside the extracellular vesicles. Although these proteins are certainly involved in the biogenesis process of extracellular vesicles, their precise function and mechanism of action requires more in-depth exploration (Kalluri and LeBleu 2020). Diverse types of RNA are present in extracellular vesicles: messenger RNA (mRNA), long non-coding RNA, and micro-RNA (miRNA). EVs can also carry soluble mediators such as cytokines. Finally, lipids such as sphingomyelin, cholesterol, phosphatidylserine, or glycosphingolipids play a major role. They form the structure of the extracellular vesicle (lipid membrane), but they can also be carried as a cargo by the extracellular vesicle (Yáñez-Mó et al. 2015). The specific biochemical composition of extracellular vesicles depends also on their parenting cell and the context in which they secrete extracellular vesicles. For example, platelets generate particles enriched in P-selectin and integrins which are crucial for coagulation, while extracellular vesicles from tumor cells contain proteolytic enzymes and metalloproteinases for the degradation of extracellular matrix (Tetta et al. 2013).
Regarding biogenesis of extracellular vesicles, three different pathways are described in the literature corresponding to the three original subtypes. The exocytosis of exosomes follows an endosomal pathway: lipids, proteins, and nucleic acids to be secreted are transported to a multivesicular body (MVB) into intraluminal vesicles (ILVs). MVBs will fuse with the plasma membrane, releasing ILVs as exosomes in the extracellular environment (Schorey et al, 2015). Microvesicles or ectosomes are directly shed from the plasma membrane. The molecular cargo follows a vertical trafficking to the plasma membrane, then by modification of membrane lipids, and budding of cytoplasmic protrusions, the vesicle is secreted (Tricarico, Clancy, and D’Souza-Schorey 2017). Finally, apoptotic bodies are secreted by cells in both pathological and physiological conditions when programmed cell death is engaged. Plasma membrane is blebbing, delivering a large vesicle into the extracellular matrix (Gustafson et al, 2017).
Once released into the extracellular environment, extracellular vesicles are transported to a target cell via body fluids. The target can be local (autocrine and paracrine effect) or far from the parent cell (endocrine effect) (Zhang et al. 2014). Even though the EV-cell interaction is still unclear and specific molecular drivers of EV-cell interaction are unknow, three general cell-EV uptake mechanisms are described in the literature. First, extracellular vesicles can have a contact with target cells by interaction of surface proteins from both extracellular vesicle and cell membranes. The second uptake mechanism is the internalization of the exosome into the cell by endocytosis (phagocytosis or micropinocytosis). The last mechanism is the fusion between exosome membrane and plasma or endosomal cell membrane (Russell et al. 2019).

Physiological and pathological functions of exosomes
Thus, extracellular vesicles are present in many body fluids such as urine, saliva, bile, or cerebrospinal fluid (CSF). They can pass across multiple barriers, even the blood-brain barrier in the central nervous system (Shetty and Upadhya 2021). Their major role is to transport molecular components from cell to cell and to protect their content from enzymes (Yáñez-Mó et al. 2015). They have multiple physiological roles such as immune regulation: they can exchange antigen information between immune cells and activate or inactivate immune cells (Robbins and Morelli 2014). They are involved in the endocrine and the nervous system as key regulators (Hanayama 2021). EVs are also known to participate in coagulation activation as they promote thrombin generation thanks to the exposure of phosphatidylserine at their surface (Berckmans et al. 2001).
Conversely, extracellular vesicles participate in the development of several diseases by propagating pathogenic molecules. In viral infections, the release mechanism and the content of extracellular vesicles is altered. Viruses use the Exosome machinery to proliferate and extracellular vesicles cargos play a role in the propagation and transmission of the virus (Kutchy et al. 2020). In Alzheimer’s and Parkinson’s diseases, extracellular vesicles propagate proteins inducing neuroinflammation and neurodegeneration (Xia et al. 2019). For instance, α-synuclein is one of the major proteins involved in the symptoms of Parkinson’s disease and in 2016, Stuendl et al. showed that CSF-derived extracellular vesicles contribute to its aggregation (Stuendl et al. 2016). Not only proteins, extracellular vesicles also carry miRNA regulating mRNAs like in Alzheimer’s disorder: a miRNA carried by extracellular vesicles is responsible of β-amyloid precursor protein accumulation through mRNA regulation (Liu et al. 2014). Moreover, the role of extracellular vesicles in cancer has been expansively described since (Zhu et al. 2012) have shown that mesenchymal stem cells (MSC)-derived exosomes promote tumor growth in vivo. Extracellular vesicles participate at every stage of cancer development in many different types of cancers (gastric cancer, breast, melanoma…) as an actor in tumorigenesis, angiogenesis, drug resistance and metastasis (Burgos-Ravanal et al. 2021).